Learning starts now
Chemistry became a whole lot easier for Grade 9
Mixing Math and Science for FUN!
What are Alkanes?
---> a kind of chemical compounds, containing the Carbon and Hydrogen atoms. To distinguish it from other hydrocarbons using its structure, look for the single bond/s. In addition, its general formula is CnH2n+2.
Example: Showing Alkane structures-in the picture below, a circle was drawn to show the single bond between a Carbon and a Chlorine (hence, the single dash).
Types of alkanes:
1. Linear alkanes
---also called skeletal alkanes, the carbons are combined together to form a zigzag line similar to a snake. See picture of octane below: Each point of zigzag, including the ends, represents carbons. Notice that there aren't any extra lines over the chain, indicationg it an alkane.
2. Branched alkanes
---are derived from linear alkanes, but instead of having just a straight chain in their chemical structure, it is branched with one or more alkyl groups. Alkyl group is a group of carbon and hydrogen atoms attached to an alkane molecule.
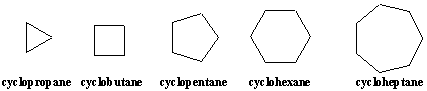
3. Cycloalkanes
---carbons and hydrogens that are joined together in single bonds to form loops.
Want to find out how to name Alkanes? Watch this video, and learn:
Reference: http://study.com/academy/lesson/alkanes-definition-properties-formula-examples.html
Credits to: owners of the pictures and video